Team members

Project
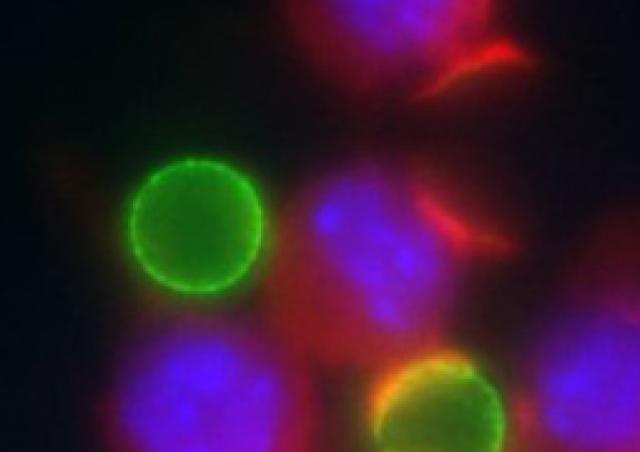
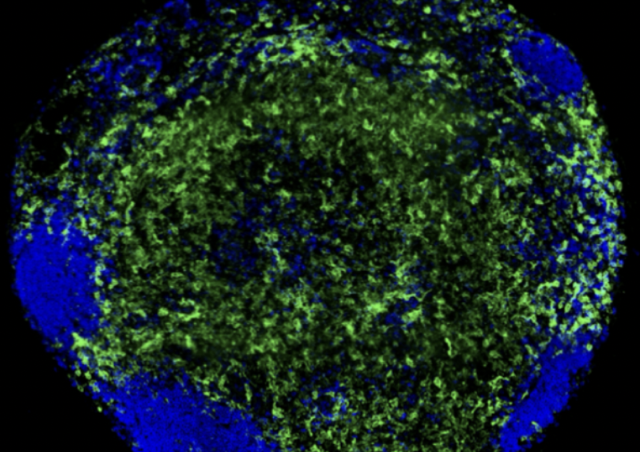
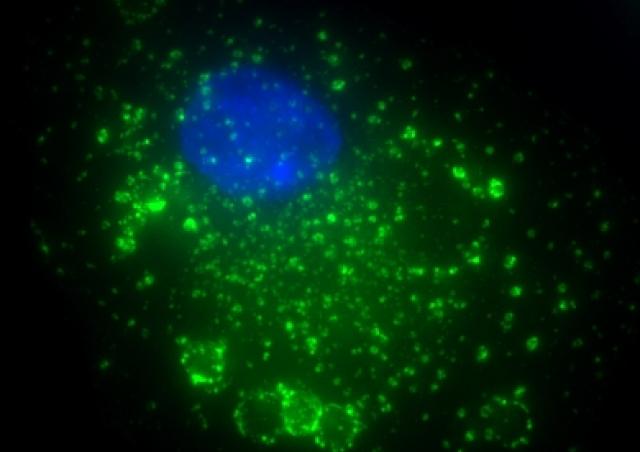
Macrophages are a major initial target of the human immunodeficiency virus (HIV) and represent a productive reservoir for the virus because of their resistance to cytopathic effects. We contributed to show that targeting the CCR5 co-receptor trafficking inhibits HIV-1 infection of macrophages (Boncompain et al., 2019). Infection of macrophages by HIV-1 causes a severe impairment of the functions of these cells, which allows the development of opportunistic pathogens. We demonstrated that macrophages infected with HIV-1 exhibit defects in phagocytosis due to the virulence factor Nef (Mazzolini et al., 2010) and defects in phagosome maturation and bacterial clearance due to the Vpr viral protein interacting with microtubule-associated motors (Dumas et al., 2015). We continue to characterize the development of opportunistic bacteria, especially invasive Salmonella Typhimurium (iNTS) strains emerged in African HIV-1-infected patients (coll Dr Jay Hinton, Dr Melita Gordon, Liverpool and Malawi) (Le-Bury et al., 2020). The complex interplay between infected cells, HIV-1 and iNTS is further analyzed in collaboration with Emmanuel Saliba and Alexander Westermann (Würzburg) with the support of Sidaction.
Viral emergence is a threat to global health. It is therefore critical to anticipate these emergences by understanding the mechanisms of the viral pathogenesis towards the development of new treatments to possibly fight off emerging diseases before they reach the pandemic stage. Yellow fever virus (YFV) represents a major health threat due to its transmission course by mosquitoes and has thus been classified as a virus with a high risk of emergence. YFV is known to replicate in liver macrophages, the Kupffer cells, but its replication in other macrophage types is still debated. Since 1968, only 43 studies have been published on the effect of YFV on macrophages phenotypes and functions. Some results have highlighted that YFV induces immune response as well as inhibit specific immune pathways in macrophages. The full extent of these modulations has yet to be discovered, especially given the lack of animal competent model recapitulating the human pathogenesis. Therefore, it is important to understand in a human macrophage model (i) what are the extent of the effects of YFV on macrophages functions and (ii) what are the mechanisms responsible for the identified modulations.
Reference
- Boncompain G, Herit F, Tessier S, […], Brelot A, Niedergang F, Perez F (2019). Differential screening identifies molecules specifically inhibiting CCR5 transport to the cell surface and HIV infection. Science Advances Oct 16;5(10):eaax0821. doi: 10.1126/sciadv.aax0821. eCollection 2019 Oct.
- Mazzolini, J., Herit, F., Bouchet, J., Benmerah, A., Benichou, S., and Niedergang, F. (2010). Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood 115, 4226-4236.
- Dumas, A., Le-Bury, G., Marie-Anais, F., Herit, F., Mazzolini, J., Guilbert, T., Bourdoncle, P., Russell, D.G., Benichou, S., Zahraoui, A., et al. (2015). The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J Cell Biol 211, 359-372.
- Le-Bury, G., Deschamps, C., Kizilyaprak, C., Blanchard, W., Daraspe, J., Dumas, A., Gordon, M.A., Hinton, J.C.D., Humbel, B.M., and Niedergang, F. (2020). Increased intracellular survival of Salmonella Typhimurium ST313 in HIV-1-infected primary human macrophages is not associated with Salmonella hijacking the HIV compartment. Biol Cell 112, 92-101.
- Locatelli, M., Faure-Dupuy, S. (2023). Virus hijacking of host epigenetic machinery to impair immune response. J Virol. 2023 Sep 28;97(9):e0065823.
- Delphin, M., Desmares, M., Schuehle, S., Heikenwalder, M., Durantel, D., Faure-Dupuy, S. (2021). How to get away with liver innate immunity? A viruses' tale. Liver Int. 2021 Nov;41(11):2547-2559.