19 members

4 PhD students

4 patents
5 projects

83 publications
Team members
Projects
Florence NIEDERGANG
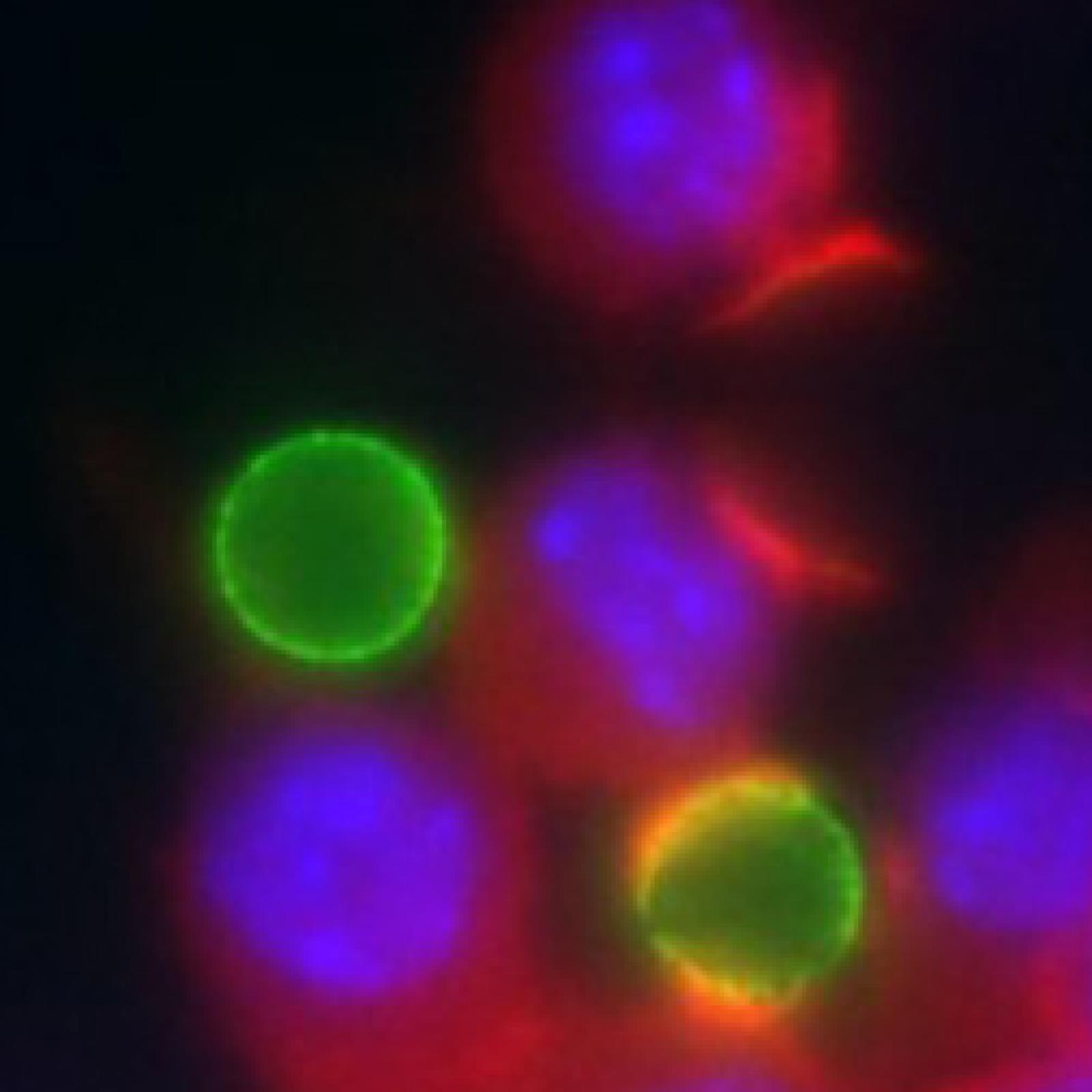
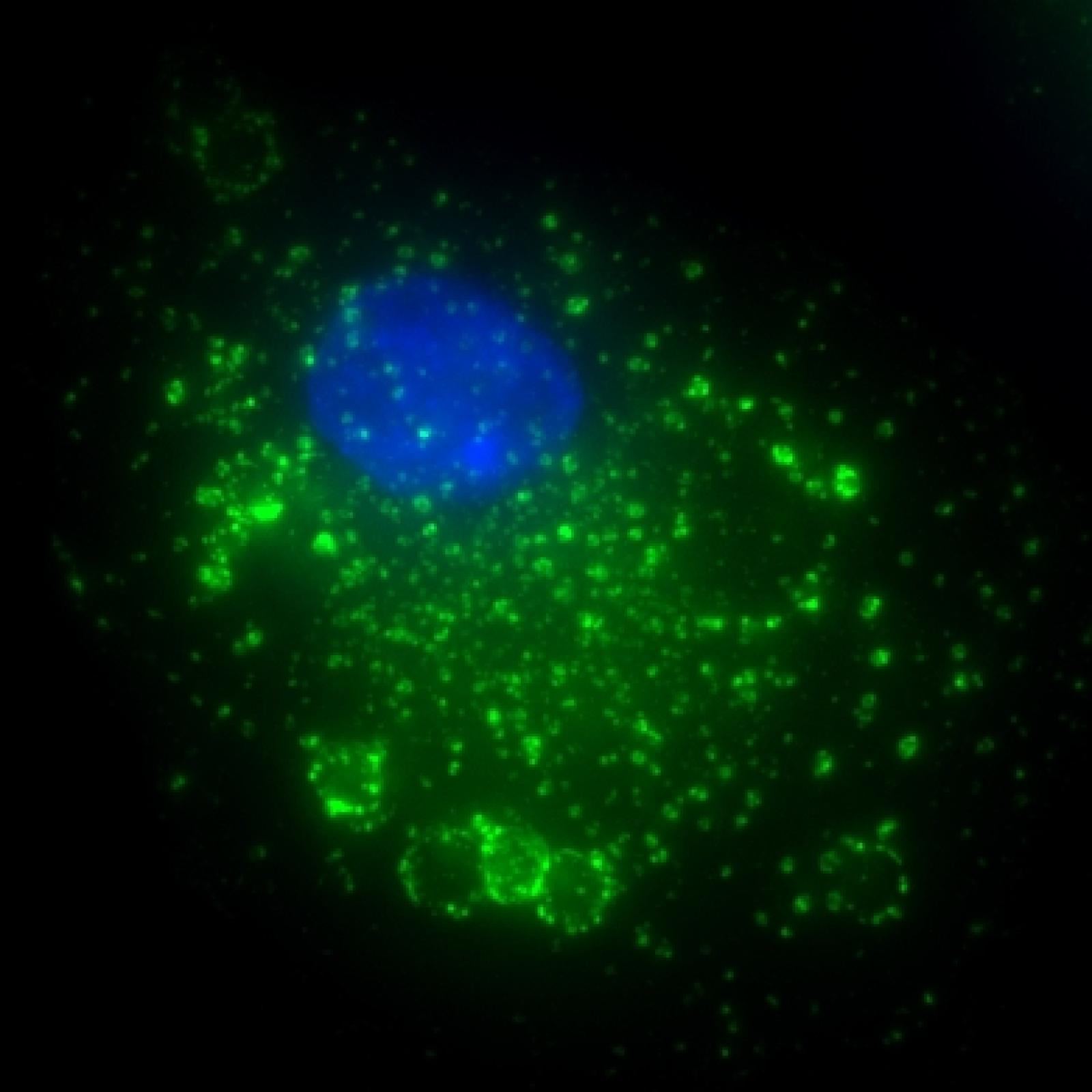
Project 1 - Mechanisms of phagocytosis in normal conditions
Florence NIEDERGANG
Suzanne FAURE-DUPUY
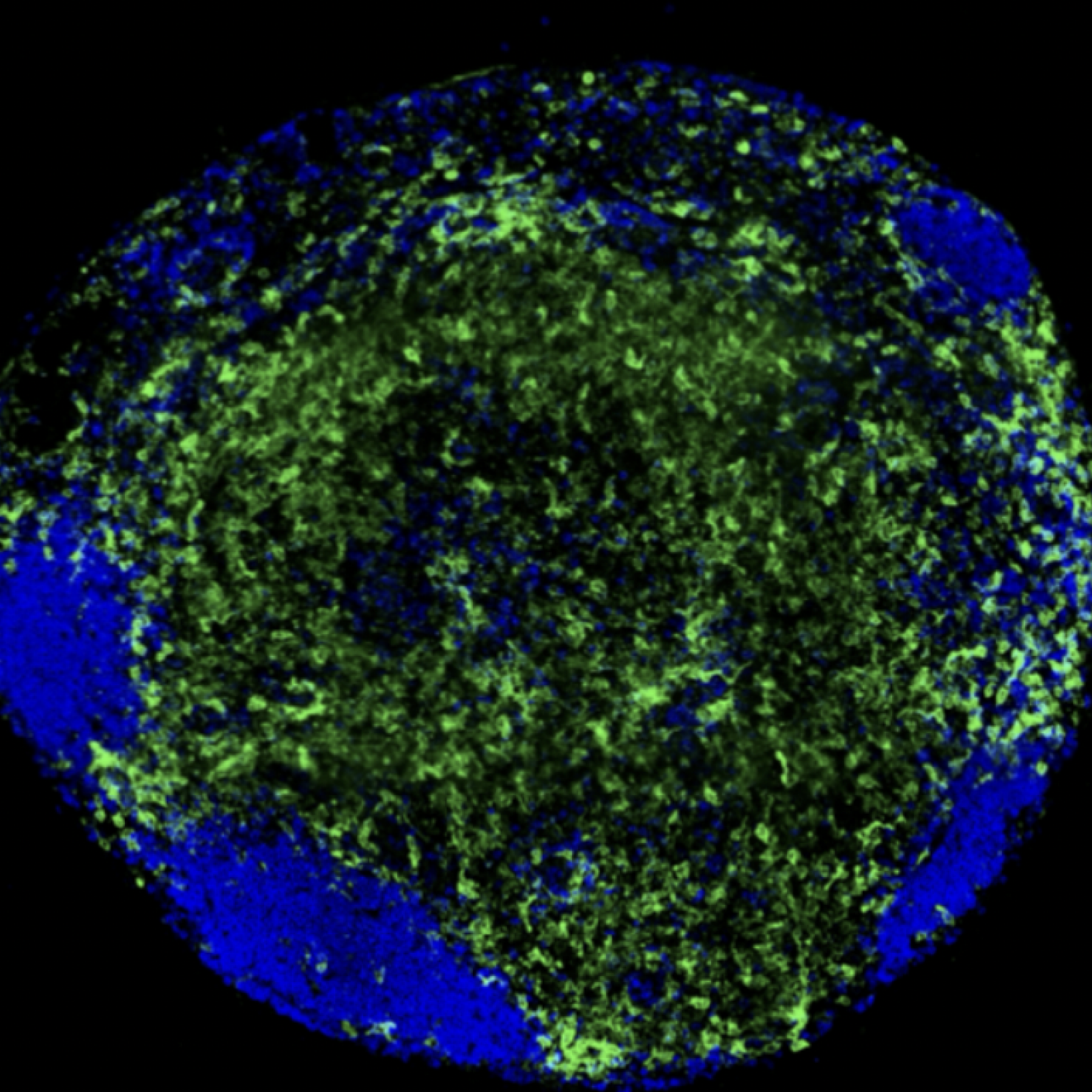
Project 3 - Perturbations of the phagocytic and activation functions of macrophages by viral infections (HIV, YFV)
Ignacio GARCIA-VERDUGO
Suzanne FAURE-DUPUY
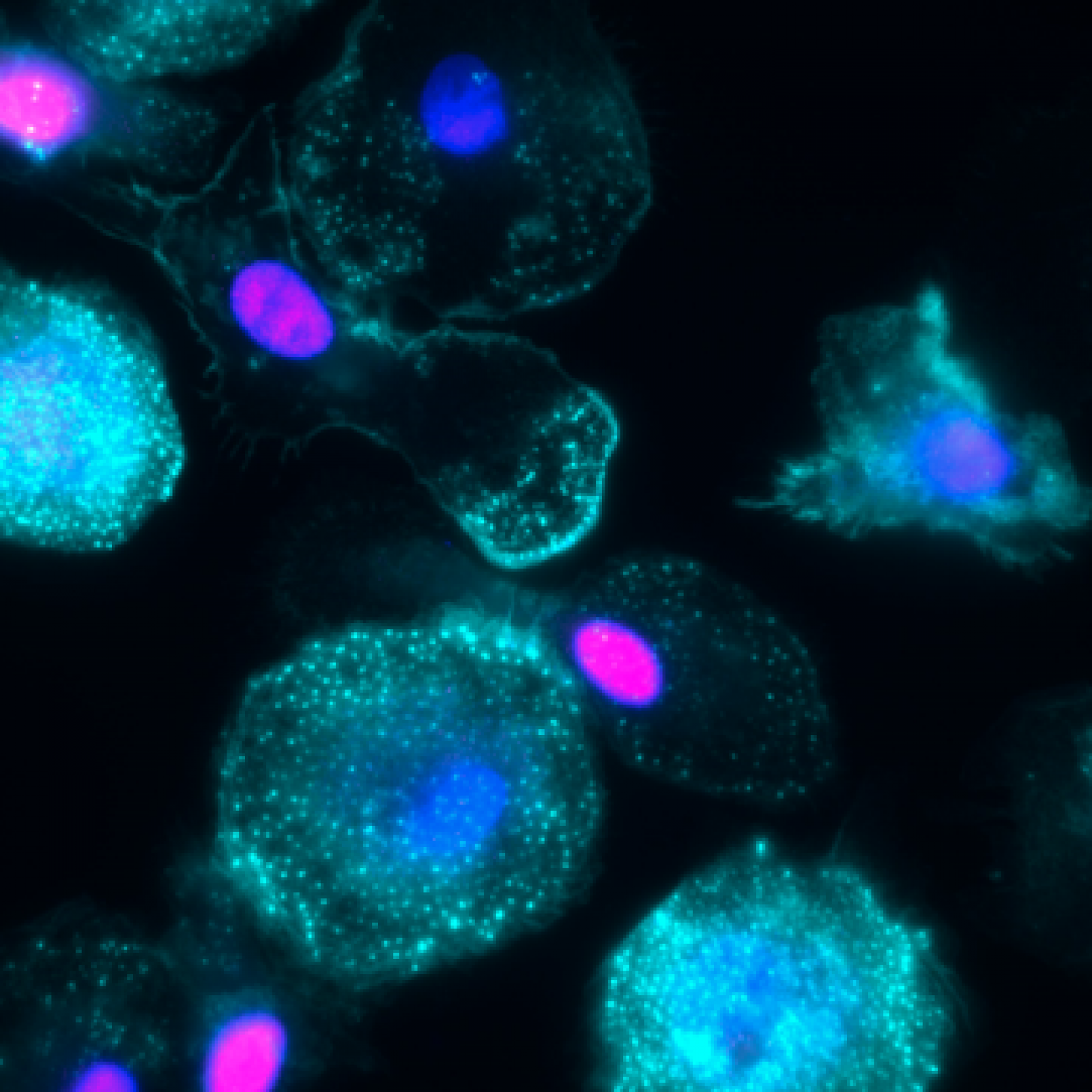
Project 4 - Perturbations of macrophages' functions by respiratory viruses
Publications
Funding
Last news from the team
Team lunch - June 2024

Team lunch - June 2023

Farewell to Tais - April 2023

Team lunch - June 2022

The team at the party for the 20th anniversary of the Institut Cochin