Project members
Project
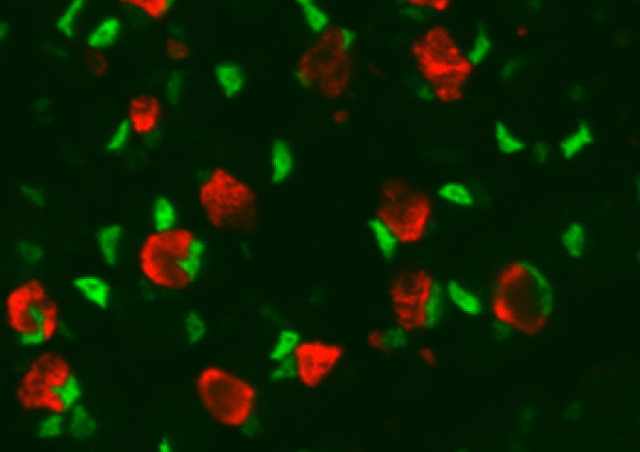
The growth of the heart during embryogenesis relies on several cell populations including cardiomyocytes (CM). Their multiplication and arrangement in compact layers to form the ventricular walls is based on several signaling pathways, some of which have already been identified. The absence of the Lrrfip2 gene leads to early embryonic death characterized by the absence of thickening of the ventricular walls. This protein is known to interact with different signaling pathways, particularly during inflammation, and we will identify which are altered in mutant CMs and are responsible for the defects observed. We have also shown that Lrrfip2 is expressed in adult muscle stem cells (satellite cells). Using conditional mutant mouse models for Lrrfip2 we will characterize its function during the process of regeneration of adult skeletal muscle in acute or chronic conditions (in an mdx mouse model deficient for the Dystrophin gene).
We are studying the role of LRRFIP2 (Leucine rich repeat in Flightless interacting protein 2) during mouse embryonic cardiac development and during adult muscle regeneration:
- Lrrfip2-/- embryos die around embryonic day 13 due to severe cardiac malformations. Through transcriptomic studies we will identify the consequences of Lrrfip2 absence in the heart of embryos mutant for Lrrfip2 and identify the signaling pathways controlled by this protein and responsible for embryonic heart growth. Cell culture experiments with wt and mutant embryonic cardiomyocytes will allow to precise the mechanisms involved.
- Lrrfip2flox/flox:Pax7CREert2/+ and mdx:Lrrfip2flox/flox:Pax7CREert2/+ animals will be treated with Tamoxifen to induce the deletion of Lrrfip2 specifically in myogenic stem cells. The consequences of absence of Lrrfip2 in the capacity of mutant myogenic stem cells to repair skeletal muscle will be estimated after acute muscle injury or in a chronic model of regeneration (mdx). Cell culture of wt and mutant cells will allow to precise the mechanisms involved.