Project members
Project
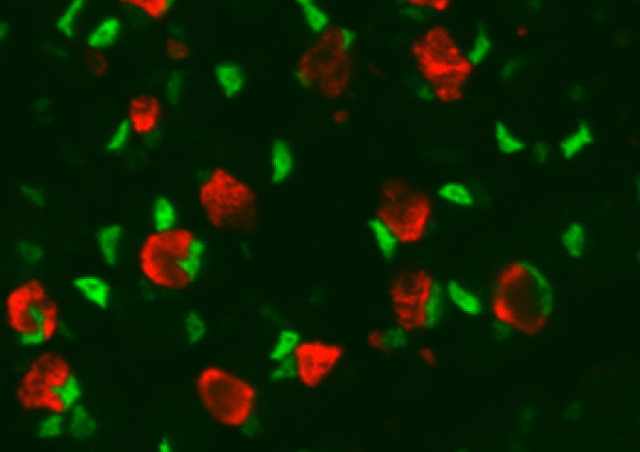
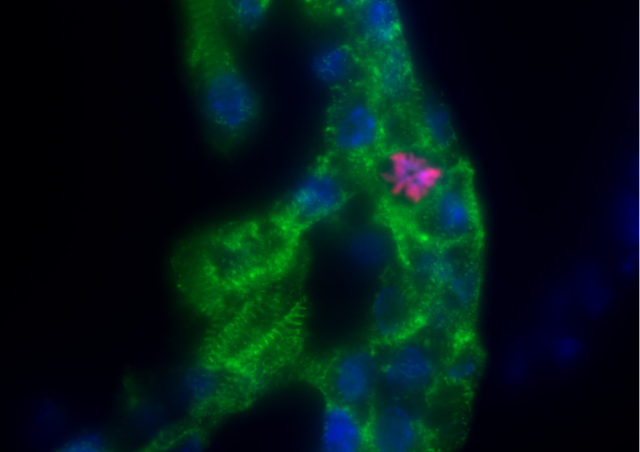
Many aspects of adult skeletal muscle plasticity including repair and growth are dependent on the fusion of muscle stem cells. Fusion involves asymmetrically distributed actin-based structures at the site of cell-cell fusion that promotes membrane apposition and may participate to the pore formation. We showed that Srf transcription factor is a key regulator of myoblast fusion in mammals required in both fusion partners by controlling the formation of actin-based finger-like protrusions. Using adult mice muscles harboring a conditional deletion of Srf in the SCs, we showed that hypertrophic muscle growth and muscle regeneration were compromised. The main goal of this project is to unravel new molecules involved in the control of biomechanical properties of myoblast fusion, in particular downstream of Srf. The second axis of our project consists at providing insights into the spatial positioning of newly recruited nuclei within myofibers upon overload-induced hypertrophy by defining whether the fusion events of muscle-lineage cells occur randomly or preferentially in muscle regions and how it is controlled using Cell Biology and lineage tracing approaches. In addition, as very little is known about the transcriptional program of newly recruited myonuclei as compared to already existing myonuclei, using snRNA-seq, we will define the transcriptional/epigenetic signature of newly-fused myonuclei that may underlie to of some aspects of “muscle memory” concept.