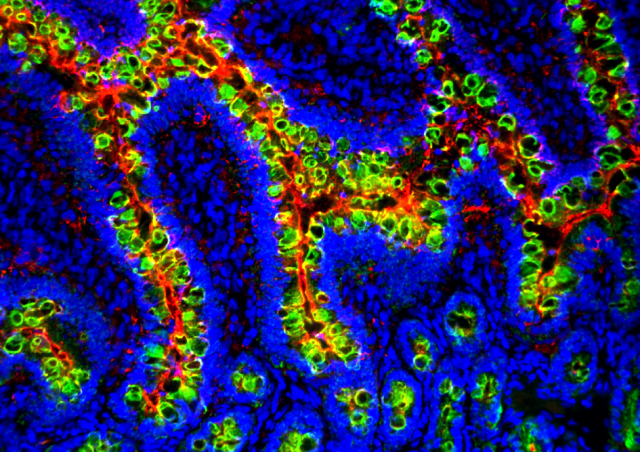
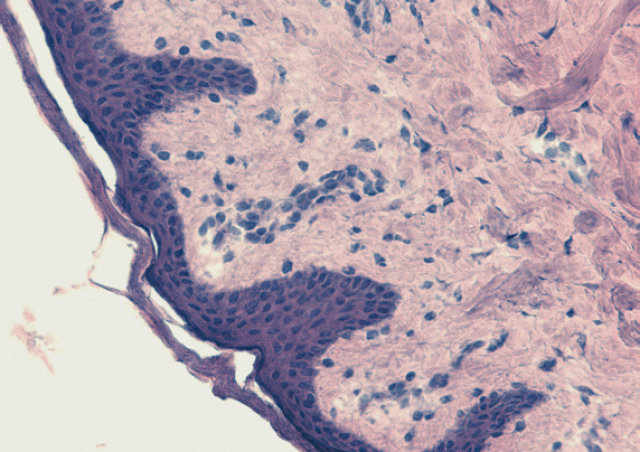
As in many viral infections, the acute phase of SARS-CoV-2 infection is characterized, in symptomatic patients, by more or less severe lymphopenia. In March 2020, by analyzing chest CT-scans of patients hospitalized in ICU for acute respiratory distress syndrome associated with COVID-19, radiologists at the Clinique Ambroise Paré in Neuilly observed that most presented with thymic hyperplasia. Its intensity seemed to be associated with greater lung damage but also with a better prognosis. Following this observation, we analyzed thymic function in about twenty of these patients and showed that it was stronger than that of the control subjects hospitalized in the same ICU, whatever the age of the patients (Cuvelier et al. 2021, Roux et al 2023). In 2023, we demonstrated that genetic marker, associated with a stonger thymic function in healthy individuals, was linked with stronger and more persistent anti-SARS-CoV-2 immune responses and to a less severe lung disease (Roux et al. 2023).
These studies clearly indicates that elevated thymic function is beneficial in patients with a severe form of COVID-19, especially in older subjects. We have shown that the extent of thymic export in patients correlates with thymus hyperplasia, lymphopenia and IL-7 plasma concentration, the essential cytokine for T-cell development in the thymus and peripheral T-cell homeostasis, that we have also identified as part of the danger signals produced rapidly during mucosal viral infections (Beq et al. 2009; Ponte et al. 2017). Our data demonstrate that a strong capacity to produce new T cells during the acute phase of SARS-CoV-2 infection increases, in some patients, the capacity to mount an effective adaptive immune response and fight lung inflammation. Furthermore, we demonstrated that a genetic trait, associated with higher thymic function in healthy individuals, determines the intensity of the anti-SARS-Cov-2 immune response in patients hospitalized for severe COVID-19, with, as a consequence, less severe disease and better prognosis.
Our expertise in measuring thymic function has also been used for many collaborative projects both in France and abroad. We have contributed to exploring the importance of this function in patients after transplantation of hematopoietic stem cells, bone marrow, heart or thymus, in subjects thymectomized in childhood, in children with chronic granulomatous disease or suffering from congenital immunodeficiency (CD4 lymphocytopenia, growth hormone deficiency, FOXN1-deficiency, DiGeorge syndrome, orphan diseases, etc.) and in patients infected with HIV-1 or HIV-2.
Project members
References
Roux HM, Marouf A, Dutrieux J, Charmeteau-De Muylder B, Figueiredo-Morgado S, Avettand-Fenoel V, Cuvelier P, Naudin C, Bouaziz F, Geri G, Couëdel-Courteille A, Squara P, Marullo S, Cheynier R. Genetically determined thymic function affects strength and duration of immune response in COVID patients with pneumonia. Sci Adv. 2023 Sep 22;9(38)
Cuvelier P, Roux H, Couëdel-Courteille A, Dutrieux J, Naudin C, Charmeteau de Muylder B, Cheynier R, Squara P, Marullo S. Protective reactive thymus hyperplasia in COVID-19 acute respiratory distress syndrome. Crit Care. 2021 Jan 4;25(1):4.
Dion ML., Bordi R., Zeidan J., Asaad R., Boulassel MR., Routy JP., Lederman MM., Sékaly RP. and Cheynier R. (2007). Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood, 109: 2912-2920.
Cavazzana-Calvo M., Carlier F, Le Deist F., Morillon E., Taupin P., Gautier D., Radford-Weiss I., Caillat-Zucman S., Neven B., Blanche S., Cheynier R., Fischer A. and Hacein-Bey-Abina S. (2007). Long-term T cell reconstitution after haematopoietic stem cell transplantation in primary T cell immunodeficient patients is associated with myeloid chimerism and possibly the primary disease phenotype. Blood 109(10):4575-81.
Gautier D., Beq S., Cortesão C., Sousa A.E. and Cheynier R. (2007). Efficient thymopoiesis participates in the maintenance of peripheral CD4 T-cells during chronic HIV-2 infection. Journal of Virology 81: 12685-8.
Castermans E., Baron F., Willems E., Schaaf-Lafontaine N., Meuris N., Gothot A., Vanbellighen J-F., Herens C., Seidel L.,Geenen V., Cheynier R. and Beguin Y. (2008). Evidences for neo-production of T-cells by the thymus after nonmyeloablative conditioning. Haematologica, 93(2): 240-7.
Dulude G.*, Cheynier R.*, Gauchat D., Abdallah A., Kettaf N., Sékaly R.-P. and Gratton S. (2008). The magnitude of thymic output is genetically determined through controlled intrathymic precursor T cell proliferation. Journal of Immunology 181:7818-24.
Morrhaye G, Kermani H, Legros JJ, Baron F, Beguin Y, Moutschen M, Cheynier R, Martens HJ, Geenen V. (2009). Impact of growth hormone (GH) deficiency and GH replacement upon thymus function in adult patients. PLoS ONE. May 22;4(5):e5668.
Albuquerque A., Marques J., Silva S.L., Ligeiro D., Devlin B-H., Dutrieux J., Cheynier R., Pignata C., Victorino R., Markert M.L., Sousa A.E. (2012). Human FOXN1-Deficiency Is Associated with αβ Double-Negative and FoxP3+ T Cell Expansions that Are Distinctly Modulated upon Thymic Transplantation. PloS One. 7(5): e36651.
Darlington P.J., Touil T., Doucet J.S., Gaucher D., Zeidan J., Gauchat D., Corsini R., Kim H.J., Duddy M., Jalili F., Arbour N., Kebir H., Chen J., Arnold D.L., Bowman M., Antel J., Prat A., Freedman M.S., Atkins H., Sekaly R., Cheynier R., Bar-Or A.; Canadian MS/BMT Study Group. (2013) Diminished Th17 (not Th1) responses underlie multiple sclerosis disease abrogation after hematopoietic stem cell transplantation. Ann Neurol. Mar;73(3):341-54.
Régent A, Autran B, Carcelain G, Cheynier R, Terrier B, Charmeteau-De Muylder B, Krivitzky A, Oksenhendler E, Costedoat-Chalumeau N, Hubert P, Lortholary O, Dupin N, Debré P, Guillevin L, Mouthon L; French Idiopathic CD4 T Lymphocytopenia Study Group. (2014) Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow-up of 40 patients. Medicine (Baltimore). 2014 Mar;93(2):61-72.
Albuquerque A., S. Fernandes, R. Tendeiro, R. Cheynier, M. Lucas, S. Silva,R. Victorino and A. Sousa. (2017) Major CD4 T-cell depletion and immune senescence in a patient with Chronic Granulomatous Disease. Frontiers in Immunology, 11 May 2017.
Silva SL, Albuquerque A, Amaral AJ, Li QZ, Mota C, Cheynier R, Victorino RMM, Pereira-Santos MC, Sousa AE. (2017) Autoimmunity and allergy control in adults submitted to complete thymectomy early in infancy. PLoS One. Jul 7;12(7):e0180385.
Sannier A, Stroumza N, Caligiuri G, Le Borgne-Moynier M, Andreata F, Senemaud J, Louedec L, Even G, Gaston AT, Deschildre C, Couvelard A, Ou P, Cheynier R, Nataf P, Dorent R, Nicoletti A. (2017) Thymic function is a major determinant of onset of antibody-mediated rejection in heart transplantation. Am J Transplant. 2018 Apr;18(4):964-971.
Rb-Silva R, Nobrega C, Azevedo C, Athayde E, Canto-Gomes J, Ferreira I, Cheynier R, Yates AJ, Horta A, Correia-Neves M. (2019) Thymic Function as a Predictor of Immune Recovery in Chronically HIV-Infected Patients Initiating Antiretroviral Therapy. Front Immunol. 2019 Feb 5;10:25.
Massey J, Jackson K, Singh M, Hughes B, Withers B, Ford C, Khoo M, Hendrawan K, Zaunders J, Charmeteau-De Muylder B, Cheynier R, Luciani F, Ma D, Moore J, Sutton. I. (2022) Haematopoietic Stem Cell Transplantation Results in Extensive Remodelling of the Clonal T Cell Repertoire in Multiple Sclerosis. Front Immunol. 2022 Feb 7;13:798300. doi: 10.3389/fimmu.2022.798300.