Project members
Project
It is now well admitted that neutrophils are involved in numerous non infectious conditions including cancer, autoimmune, cardiovascular and allergic diseases.
During the last decades, new concepts have dramatically modified the image of the neutrophils: (1) Neutrophils are now endowed of plasticity with the generation of new sub-populations of neutrophils with diverses immunomodulatory functions; (2) Neutrophils can do reverse migration; (3) Neutrophils can communicate and instruct various immune cells; (4) Neutrophil death is complex and is a very important determinant in the immune and inflammatory response (Figure 1).


It is now evident that there is a tight link between death pathways that are activated in neutrophils and inflammation resolution.
Historically, neutrophils have been viewed only as professional phagocytic cells that are able to phagocytose and destroy infectious agents. Indeed, they are key anti-infectious actors in host defense but can mediate tissue damages (Witko-Sarsat et al Lab Invest 2000).
However, it is now clear that the role of neutrophils go far beyond phagocytosis and pathogen killing. Following successive steps of adhesion, chemotaxis and migration, can phagocytose invading pathogens and mobilize their microbicidal effector molecules, including reactive oxygen species, antibiotic proteins and proteinases. This neutrophil activation triggers an apoptosis program to ultimately signal their phagocytosis to macrophages (Witko-Sarsat et al Trends Immunol 2011).
The recognition and the subsequent engulfment of apoptotic neutrophils by macrophages is a key event of the resolution of inflammation, which can be associated with autoimmunity or inflammatory. In addition, this safe disposal of apoptotic neutrophils generate anti-inflammatory signals to complete the inflammation resolution (Thieblemont et al seminar Immunol 2017 ; Thieblemont et al Eur J Clin Invest. 2018)
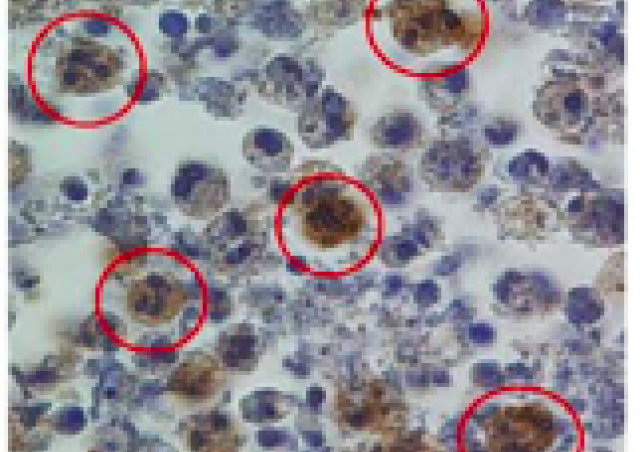
In contrast, necroptosis, the death induced by death receptors when apoptosis is inhibited, is an inflammatory death. It is mediated by the necrosome, an oligomer composed of RIPK1, RIPK3 and MLKL leading to the cell lysis. More recently, the pyroptosis, another inflammatory death induced by inflammasomes has been characterized. Mechanisms leading to the activation of the inflammasome NLRP3 and to the secretion of interleukine-1beta has been extensively studied in monocytes/macrophages. Less attention has been paid to inflammasome in neutrophils which seem to have specific signaling pathways (Figure 2).
FADD : a regulator of apoptosis and other death pathways
The role of FADD protein is well known to regulate apoptosis-related pathways but its biologic function extends beyond apoptosis to other death pathways. FADD is crucial for numerous biologic processes such as embryogenesis, survival, proliferation, cell cycle progression, autophagy, innate immunity, inflammation and cancer (Tourneur et al. Medical Immunol 2005; Tourneur and Chiocchia. Trends Immunol 2010; Vilmont et al. Rheumatology 2012; Mouasni and Tourneur. Trends Immunol 2018).

In a recent study, the group of Lea Tourneur has shown that FADD was secreted in plasma membrane-derived microvesicles and that this secretion was associated to the inflammatory relapses in rheumatoid arthritis.

PCNA (Proliferating Cell Nuclear Antigen) : an unexpected actor in neutrophil survival
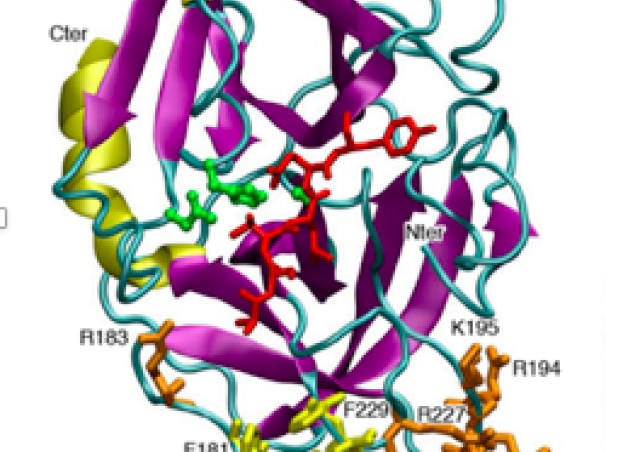
In neutrophils, PCNA is a trimeric protein (in blue on the cartoon) which can build a scaffold by its association with several proteins (Witko-Sarsat et al. J Exp Med 2010). In the neutrophil, PCNA is exclusively cytosolic and its scaffold is adapted to the different states of neutrophil physiology:
1) Under the steady state survival (represented in the center), PCNA is associated with “steady-state partners” such as pro-apoptotic proteins (procaspases) 2) Under inflammation or G-CSF treatment or hypoxia, de novo synthesis of “inducible partners” (for exemple p21/waf1) will modify the scaffold to promote survival. 3) Under non-inflammatory conditions, physiologic apoptosis is accompanied by a proteasomal degradation of PCNA with caspase activation. 4) Phagocytosis also modulates the PCNA scaffold but the role of PCNA in neutrophil fate seems to be highly dependent on the type of bacteria phagocytosed.
References : Witko-Sarsat V & Ohayon D. Proliferating cell nuclear antigen in neutrophil fate. Immunol Rev. 2016 Sep;273(1):344-56.


PCNA is a new regulator of the NADPH oxidase
Recently we have uncovered a new function of PCNA in the control of NADPH oxidase : PCNA can bind the p47phox protein thereby controlling the production of ROS (Ohayon et al J Exp Med 2019). This work has shown for the first time that it was possible to obtain a strong anti-inflammatory effect by targeting the association between PCNA and p47phox in neutrophils in a colitis model in mice.
PCNA: a new marker of resistance to chemotherapy in leukemia
We have also shown that the exclusive cytosolic localisation of PCNA results from an active nuclear export controlled by the CRM1 exportin. We have identified the NES in PCNA sequence which is located at the inner face of the trimer and is accessible to the exportin only when PCNA is monomeric. (Bouayad et al J Biol Chem 2012; De Chiara et al J Leukocyte Biol 2013).
This nuclear-to-cytosolic relocalization occurred which occured at the end of granulocyte differentiation under normal conditions, is observed in the promyelocytic HL-60 cells resistant to daunorubicin to favor their survival. Cytosolic PCNA could constitute a novel therapeutic target or a marker of response to treatment.