Team members
Projects
Ralf JOCKERS
Deciphering the function of melatonin receptors
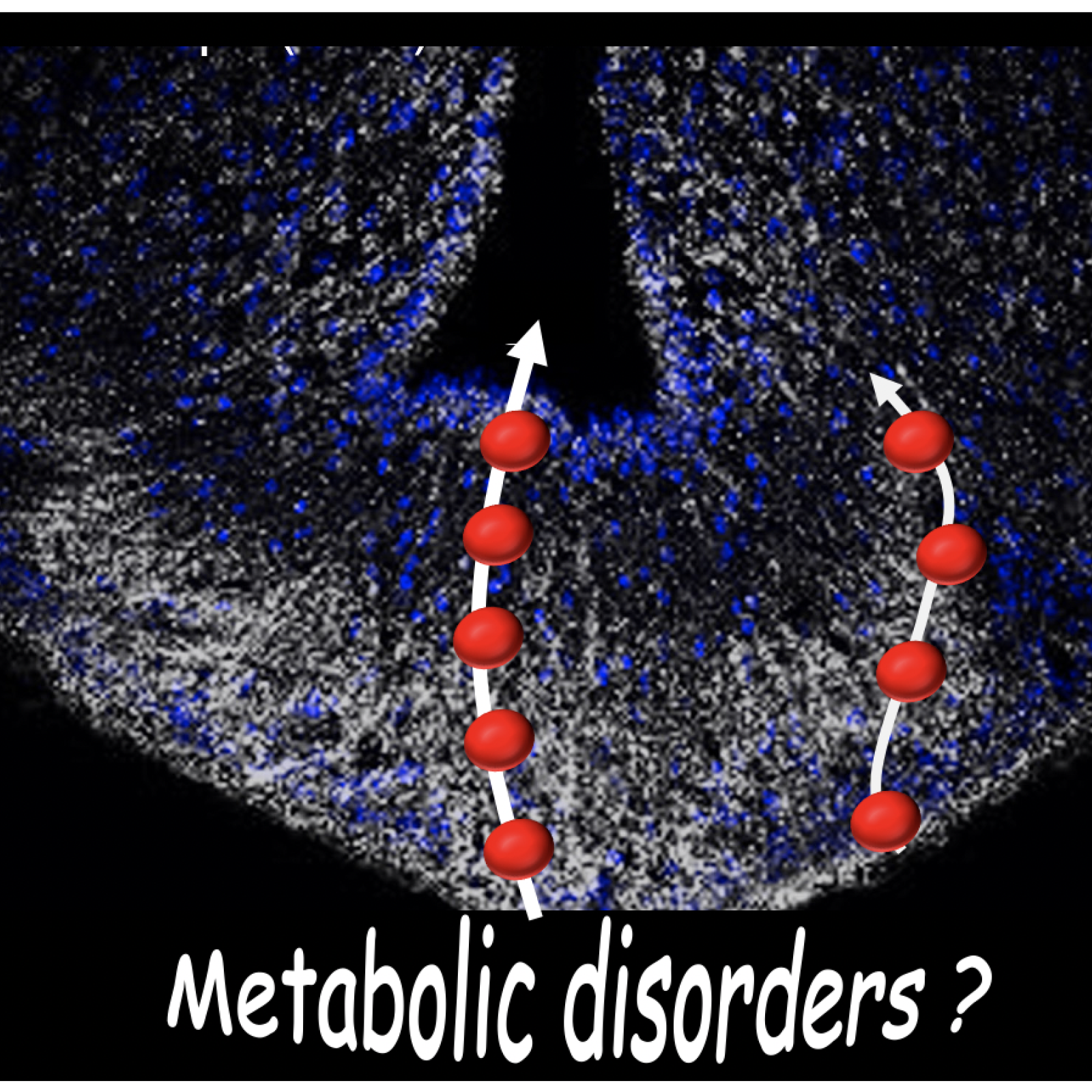
Julie DAM
Ralf JOCKERS
Deciphering the function of GPR50 receptor in health and diseases
Julie DAM
Ralf JOCKERS
Identify new GPCR genes associated with obesity and Type 2 diabetes
5 Main publications of the team in the last 5 years
1. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Duquenne M, Folgueira C, Bourouh C, Millet M, Silva A, Clasadonte J, Imbernon M, Fernandois D, Martinez-Corral I, Kusumakshi S, Caron E, Rasika S, Deliglia E, Jouy N, Oishi A, Mazzone M, Trinquet E, Tavernier J, Kim YB, Ory S, Jockers R, Schwaninger M, Boehm U, Nogueiras R, Annicotte JS, Gasman S*, Dam J*, Prévot V*. Nat Metab 2021
Aug; 3(8):1071-1090. doi: 10.1038/s42255-021-00432-5. Epub 2021 Aug 2. PMID: 343415682. SARS-COV-2 spike binding to ACE2 in living cells monitored by TR-FRET. Cecon E, Burridge M, Cao L, Carter L, Ravichandran R, Dam J*, Jockers R*. Cell Chem Biol 2022 Jan 20;29(1):74-83.e4. doi: 10.1016/j.chembiol.2021.06.008. Epub 2021 Jul 2. PMID: 34246414
3. Novel repertoire of tau biosensors to monitor pathological tau transformation and seeding activity in living cells. Cecon E, Oishi A, Luka M, Ndiaye-Lobry D, François A, Lescuyer M, Panayi F, Dam J, Machado P, Jockers R. Elife 2023 Mar 14;12:e78360. doi: 10.7554/eLife.78360. PMID: 36917493
4. Mitochondria-targeted melatonin photorelease supports the presence of melatonin MT1 receptors in mitochondria inhibiting respiration. Somalo-Barranco G, Pagano Zottola AC, Abdulrahman AO, El Zein RM, Cannich A, Muñoz L, Serra C, Oishi A, Marsicano G, Masri B, Bellocchio L, Llebaria A, Jockers R. Cell Chem Biol 2023 Aug 17;30(8):920-932.e7. doi: 10.1016/j.chembiol.2023.07.009. PMID: 37572668
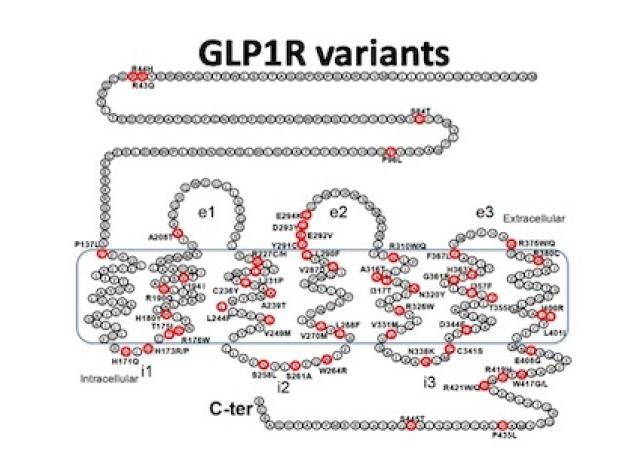
5. Human GLP1R variants affecting GLP1R cell surface expression are associated with impaired glucose control and increased adiposity. Gao W, Liu L, Huh E, Gbahou F, Cecon E, Oshima M, Houzé L, Katsonis P, Hegron A, Fan Z, Hou G, Charpentier G, Boissel M, Derhourhi M, Marre M, Balkau B, Froguel P, Scharfmann R, Lichtarge O, Dam J, Bonnefond A, Liu J, Jockers R. Nat Metab 2023 Sep 14. doi: 10.1038/s42255-023-00889-6.