Team members
The team “Metabolism, Immunity, Cancer and Therapies”, created in 2024, focuses on the molecular basis of energy metabolism in cancers (hepatocellular carcinoma, colorectal cancer, lung cancer and sarcoma). Our project supports strong translational and clinical research to accelerate the transfer of advances in basic research for the benefit of individuals and patients. Our team brings together experts in oncology, metabolism, immunology, hepatology, gastroenterology, physiology, nutrition, molecular and cell biology, and genomics.
Metabolism and cancer
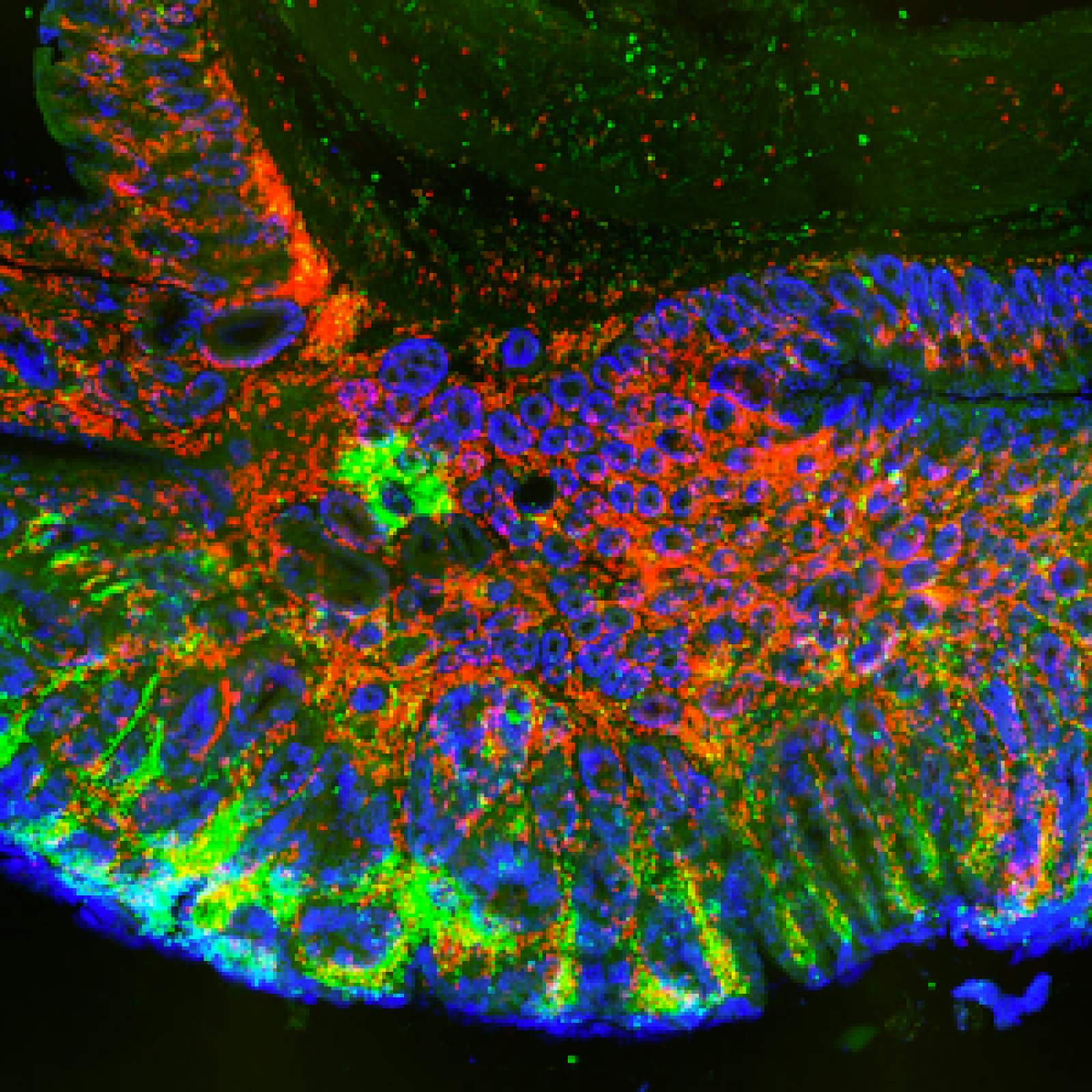
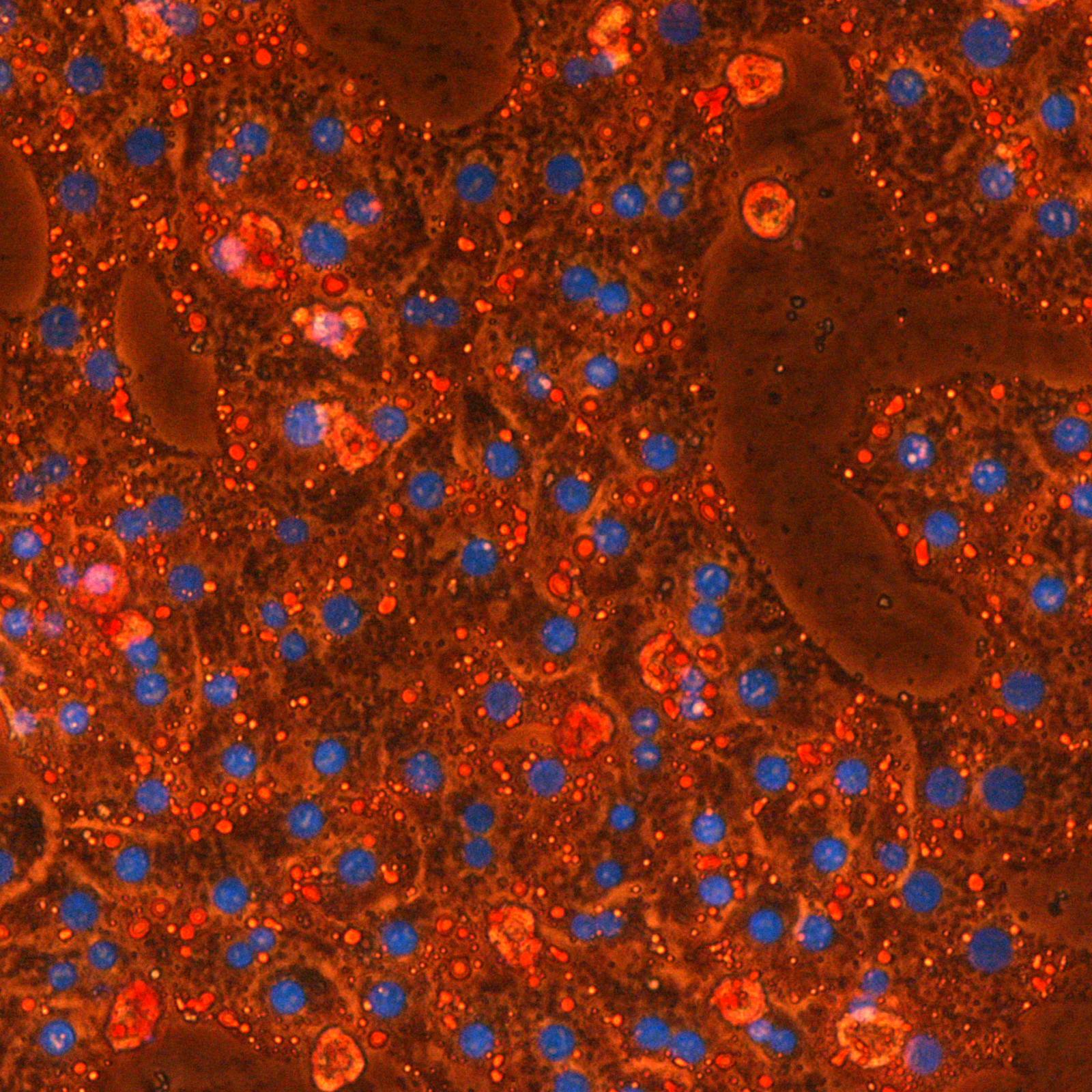
Although of diverse origins, most cancer cells share common features such as the ability to escape cell death and the use of different energy sources. In the current paradigm, aerobic glycolysis is considered the central metabolic feature of cancer cells (Warburg effect). However, recent data indicate that significant changes in other metabolic pathways are also required, notably those of amino acids, lipids and mitochondria (oxidative phosphorylation or OxPhos).
“Our research strategy is to determine whether modulating cancer cell metabolism and metabolic enzymes can modulate tumor growth, response to chemotherapy and anti-cancer immune response.”
The malignant transformation of cells is thus associated by profound metabolic changes to cope with increasing demands for biomass and energy. In this complex picture, the metabolic adaptability of the tumor could therefore constitute a weakness that could be targeted to eliminate cancer cells. In this context, the aim of our research is to understand these metabolic changes by identifying points of metabolic vulnerability specific to cancer cells, which could ultimately become effective targets for the development of new treatment strategies in oncology.
Immune microenvironment
Another area of interest for our team is the interaction of cancer cells with their environment. Our work in this area is particularly focused on immune cells (tumor-associated lymphocytes and macrophages), as they play an important role in creating a favorable or unfavorable environment for tumor development. As metabolism plays an important role in the activation of immune cells, we are seeking to understand these metabolic interactions and to find other immune cell-dependent entry points for targeting cancer cells.
Overall, our laboratory studies the molecular basis of tumor metabolism in cancer: how it can be targeted and how it can influence the anti-cancer immune response along a continuum from fundamental mechanisms to the clinic. We use stable isotopic tracers to identify the dynamics of cellular metabolism. We are also developing unique preclinical models to study the metabolic dependence of tumor cells when in their appropriate microenvironment.
Projects
Renaud DENTIN
Clotilde ALVES-GUERRA
Energy metabolism, oncogenesis and therapies
Publications
Fundings
Last news ...
Contacts
Renaud Dentin
Institut Cochin
INSERM U1016, CNRS UMR8104, University Paris-Cité
Faculté de Médecine - 3ème étage - Pièce 3516
24 rue ud Faubourg Saint-Jacques
75014 Paris, France
Clotilde Alves-Guerra
Institut Cochin
INSERM U1016, CNRS UMR8104, university Paris-Cité
Faculté de Médecine - 3ème étage - Pièce 3516
24 rue ud Faubourg Saint-Jacques
75014 Paris, France