Project members
Project
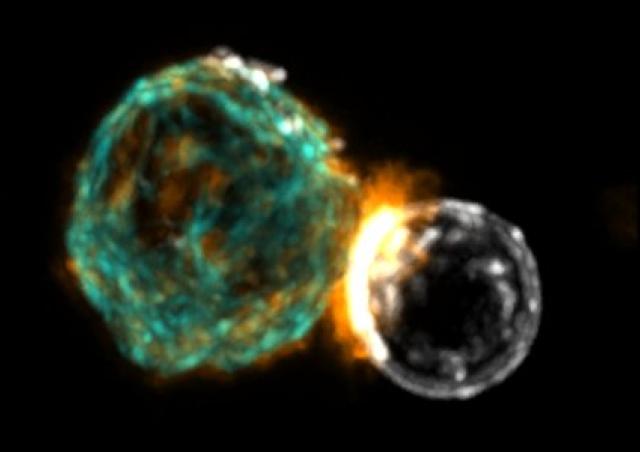
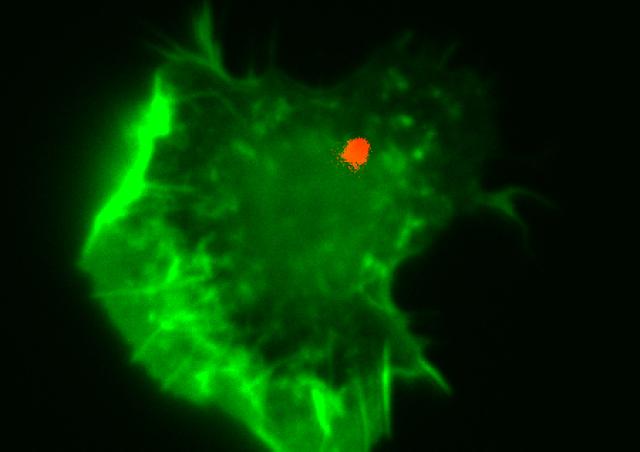
The immune system that protects us from infection and cancer is made up of specialised cells that are particularly mobile and quick to react. To fulfil their role as sentinels in the body, immune cells are constantly patrolling in the blood and tissues. This mobility is made possible by rapid and reversible changes in the morphology of immune cells such as T cells. These changes in shape are made possible by alterations in the polymerisation state of certain elements of the cytoskeleton such as actin filaments and microtubules.
Our research group is interested in the RHO GTPase signalling pathways that primarily control cytoskeletal alterations to allow immune cells to fully perform their functions of migration, activation, proliferation and differentiation. Within the RHO GTPase family, we are particularly interested in the ubiquitous members RHOA, RAC1 and CDC42. These RHO GTPases can in fact be stimulated by a large number of surface receptors engaged on immune cells in response to various stimuli present in the environment: chemokines, cytokines, inflammatory agents, antigens, bacteria...
Thus, RHO GTPases participate in the proper functioning of the immune system, and their functions altered by genetic mutations or targeted by bacterial agents can lead to numerous pathologies.

Impact of RHO GTPases in the physiology of immune cells
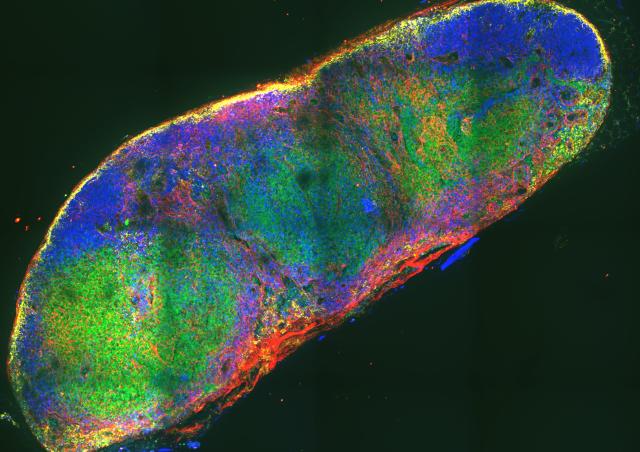
The overall objectives of our work is to determine the role of RHO GTPases in various cellular functions and to understand to what extent RHO GTPases also modulate other major signalling pathways to generate a complex and adapted cellular response.
In this context, in recent years, we have focused our studies on the CDC42 GTPase whose role in cell polarity mechanisms has been shown in many different cell types. A patient with a severe autoinflammatory syndrome carrying a mutation in the CDC42 gene has allowed us to determine that the CDC42 signalling pathway is able to control the NF-kB pathway responsible for the initiation of inflammatory processes. We are now seeking to understand from a mechanistic point of view i) how CDC42 can modulate inflammation and ii) how a partner of CDC42 can be involved in these phenomena as well as in the development of different types of immune cells.
In parallel to this work, we are also interested in the modulation of the RHOA pathway by heterotrimeric G proteins activated by numerous receptors present on the surface of immune cells.
The results obtained should therefore broaden our knowledge of the role of RHO GTPases in immune cells, and open up new therapeutic perspectives.