Project members
Project
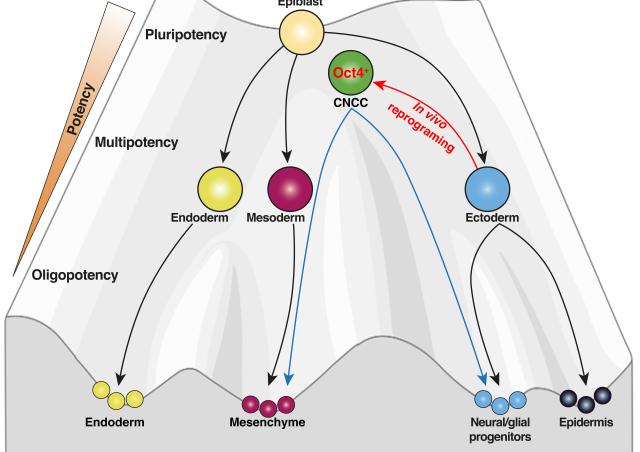
Cell diversity generally increases during vertebrate development. However, cranial neural crest cells exhibit a particular differentiation pathway, characterized by a phase of transcriptional convergence during the epithelial-mesenchymal transition.
I have shown that prior to migration, cranial neural crest cells possess molecular positional information that reflects their site of origin in the cranial neuroepithelium. However, this heterogeneity is rapidly erased, with migrating cranial neural crest cells no longer expressing anterior-posterior position genes but exhibiting a homogeneous molecular signature. This indicates that these cells acquire a certain level of plasticity, allowing them to adapt to future environmental cues during their migration.
The molecular mechanisms regulating how cranial neural crest cells decrease their cellular heterogeity and control their plasticity through rapid transcriptional and epigenetic changes remain unknown.

To understand how cranial neural crest cells maintain a high level of plasticity throughout development, we seek to decipher the transcriptional programs and chromatin rearrangements that control the establishment of molecular heterogeneity in these cells as well as those that erase it, and ultimately, how these processes influence the plasticity of this population.
To uncover programs regulating the cell fate and plasticity of this population, we combine multi-omics screens, bioinformatics analyses, and in vivo and in vitro functional assays to identify candidate genes and regulatory elements controlling the remodeling of cranial neural crest cells positional identity.
Taken together, this work will provide an understanding of the molecular mechanisms that control cranial neural crest cell positional identity remodeling and help to imagine how these processes could be redeployed to regulate cell plasticity and stimulate endogenous regeneration.