Bacterial pathogenesis and innate immune signaling
The emergence of antibiotic resistance in numerous pathogenic bacteria requires the development of innovative therapeutic strategies based on the characterization of new molecular determinants of virulence and of cellular factors involved in critical host-pathogen interactions for infection.
From this perspective, the molecular mechanisms responsible for activating and regulating innate immunity, which constitutes the first line of defence against pathogens, are of particular interest. During an infection, bacteria are detected by immune cell pathogen recognition receptors (PRRs) but also epithelial and endothelial cells which act as sentinels of immunity. After recognition of bacterial molecular patterns, PRRs trigger pro-inflammatory signalling pathways that result in the secretion of a wide range of immune effectors such as cytokines, chemokines and antimicrobial peptides.
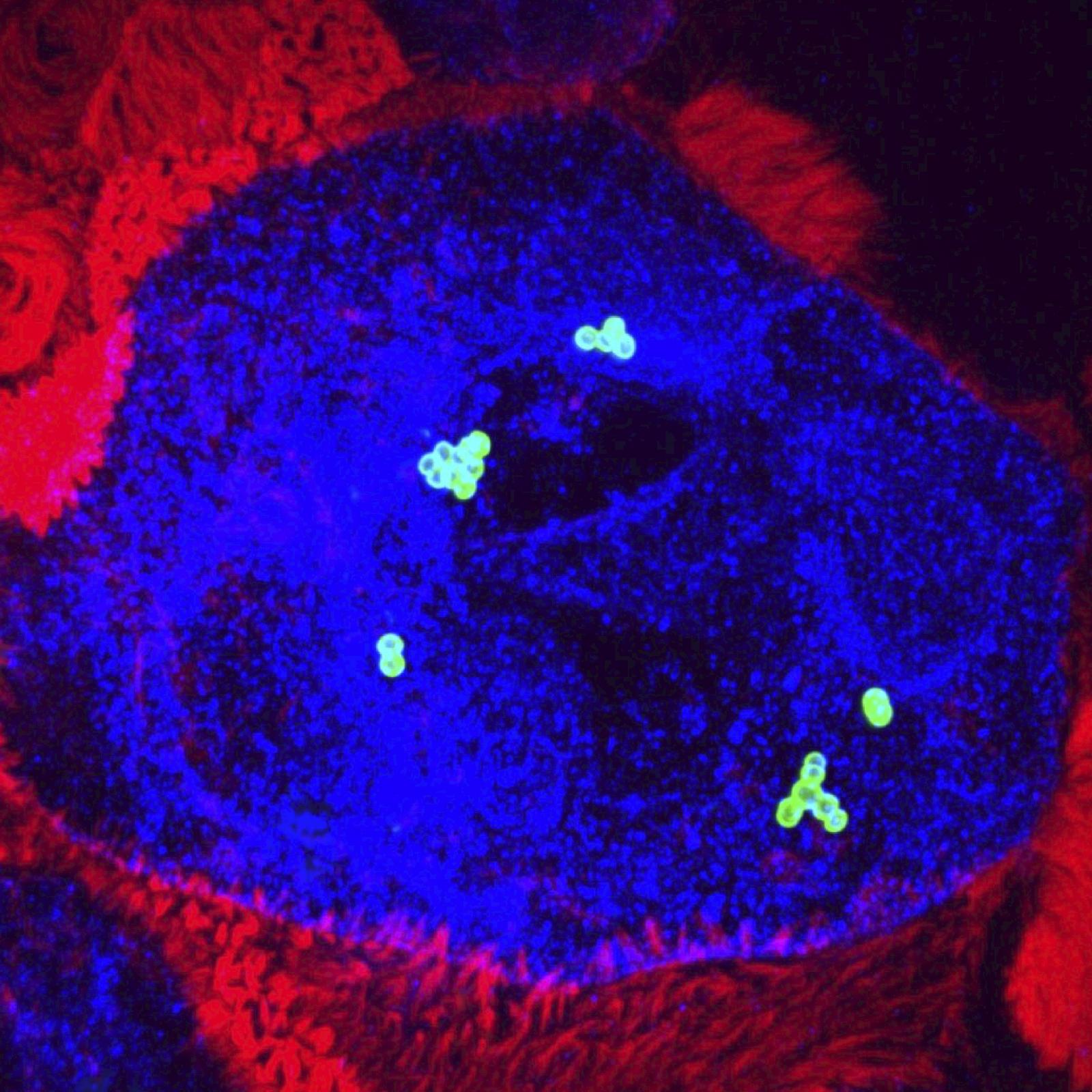
Our laboratory works at the interface between immunology and cell biology and studies the molecular mechanisms involved in the detection of infectious bacteria and the control of innate immunity. We combine RNAi screens, live cell fluorescence microscopy, electron microscopy, proteomics and transcriptomics to characterize new host-pathogen interactions that could be targeted by anti-infective treatments. The Gram-negative bacterium Shigella flexneri is one of our main models of infections.
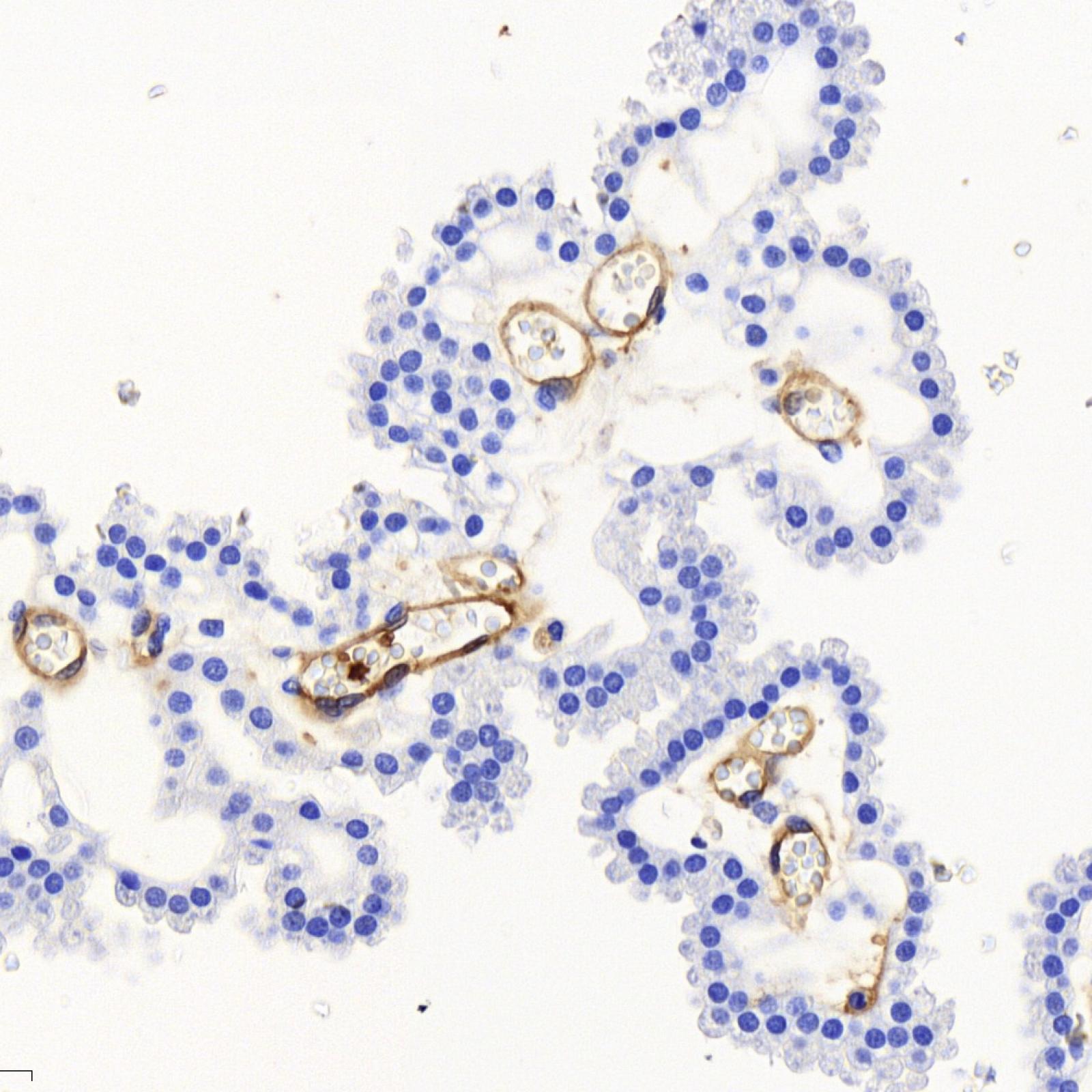
Our other model of infection is one of the two major Streptococci, Streptococci group B (GBS, Streptococcus agalactiae) which is involved in severe invasive infections occurring during pregnancy or the neonatal period.
GBS is found in the intestinal and vaginal flora of 10-30% of healthy adults. However, GBS is the leading cause of neonatal infection. Our goal is to understand why an adult commensal bacterium is a formidable pathogen of the newborn. We identify the mechanisms by which GBS can cross physiological barriers, mainly the digestive and blood-brain barriers, and escape the innate immune system. We are interested in the bacterial factors involved and the host factors with which they interact, as well as the predisposition of the infant. In this context, we study the appearance or disappearance of cellular factors and the maturation of physiological barriers during the first weeks of life. We also study the microbiota of the newborn as a function of age and environmental parameters such as the administration of antibiotics.
Finally, Claire Poyart directs the National Reference Center for Streptococci (CNR-Strep) which collects clinical data sent by a network of clinical laboratories throughout France and characterizes at the molecular level the strains responsible for invasive and non-invasive infections.