Most autoinflammatory diseases are caused by mutations in innate immunity genes. Previously, our team and others have identified mutations in the CDC42 gene in patients presenting with syndromes characterised by neonatal cytopenia and hyperinflammation, particularly in the severe forms with haemophagocytic lymphohistiocytosis and skin rashes (NOCARH syndrome). CDC42 is a ubiquitous GTPase belonging to the RHO family that controls multiple cellular functions, in particular alterations of the cytoskeleton and intracellular protein trafficking.
Our work reveals that the p.R186C recurrent variant of CDC42, trapped in the Golgi apparatus, blocks anterograde (endoplasmic reticulum (ER) - Golgi) and retrograde (Golgi - ER) protein transport. This blockade causes ER stress and COPI-dependent accumulation of STING protein in the Golgi. Interestingly, the p.192C24 variant, also trapped in the Golgi, shows similar effects, in contrast to two other CDC42 variants that exhibit normal cytosolic localisation. We also show that the two CDC42 variants trapped in the Golgi are the only ones capable of overactivating the STING pathway and IFNα production. Consistent with these in vitro and ex vivo results, the corresponding patients show elevated blood IFNα levels from the onset of the disease.
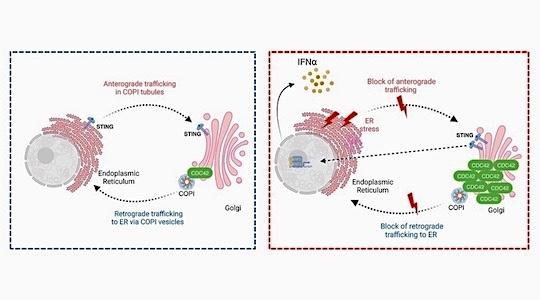
Figure: Impact of pathological CDC42 accumulation in the Golgi on intracellular protein trafficking and endoplasmic reticulum stress.
Left panel: In healthy donors, a small pool of CDC42 regulates intracellular trafficking through its interaction with COPI. Anterograde and retrograde transport of proteins between the endoplasmic reticulum (ER) and the Golgi apparatus function harmoniously to ensure the cell's homeostatic mechanisms.
Right panel: Recurrent mutations in CDC42 cause its retention in the Golgi apparatus. This generates a block in anterograde and retrograde protein trafficking, and ER stress. Consequently, it leads to Golgi accumulation of STING, excessive activation of the STING pathway and high IFNα production.
Thus, our results show that autoinflammatory patients with CDC42 trapped in the Golgi show hyperactivation of the STING-IFNα pathway in addition to increased activation of other pro-inflammatory pathways (NF-kB and Pyrin inflammasome). Our study therefore offers new avenues for combined treatments in these severe cases of autoinflammatory disease.
Reference
Iannuzzo A, Delafontaine S, El Masri R, Tacine R, Prencipe G, Nishitani-Isa M, van Wijck RTA, Bhuyan F, de Jesus Rasheed AA, Coppola S, van Daele PLA, Insalaco A, Goldbach-Mansky R, Yasumi T, Tartaglia M, Meyts I, Delon J. Autoinflammatory patients with Golgi-trapped CDC42 exhibit intracellular trafficking defects leading to STING hyperactivation and ER stress. Nat Commun. 2024 Nov 16;15(1):9940. doi: 10.1038/s41467-024-54294-y. PMID: 39550374; PMCID: PMC11569173.






