Members of the project
Project
Streptococcus pyogenes, also known as Group A Streptococcus (GAS), is a strictly human gram-positive pathogen responsible for approximately 517,000 deaths per year worldwide. GAS causes a variety of clinical manifestations, ranging from mild non-invasive infections, such as impetigo and pharyngitis, to much more severe invasive infections, such as sepsis, necrotizing fasciitis, endometritis, or streptococcal toxic shock syndrome. These infections are insidious since these repeated clinical attacks can lead to fatal post-streptococcal sequelae. There is currently no vaccine against GAS. Anti-GAS antibiotics used are broad spectrum.
The variety of infections caused by GAS is related to the fact that among the many virulence factors involved in different aspects of interaction with host tissues or resistance to the immune system, some are limited to few strains. Indeed, GAS strains are genetically very diverse. They are classified by genotyping (emm-type) and more than 200 emm-types have been identified. There is a link between the genotype and the repertoire of virulence factors and thus with tissue tropism. The emm28 genotype, prevalent in Europe and North America, is associated with gyneco-obstetrical infections. In France, these represent 60% of invasive GAS infections in women of childbearing age.
We are particularly interested in the early stages of GAS infections, corresponding to tissue adhesion, bacterial growth and tissue invasion, with endometritis as a study model. We use an emm28 clinical isolate originating from an endometritis that is genetically and phenotypically representative of emm28 strains.
Molecular basis of GAS colonization and invasion of human tissues
The project is divided into two main axes. In the first axis, we analyze the interaction of GAS with the decidua, the tissue that lines the endometrium at the end of pregnancy. It is composed of stromal cells, differentiating into decidual cells during the menstrual cycle, and immune cells, that vary during pregnancy. It is the target of nidation but also of endometrial infections. In the second axis, we study the link between fatty acid metabolism and GAS virulence.
GAS-decidua-interaction
The emm28 strains have a specific adhesin, R28, on their surface. In an original way for a GAS adhesin, R28 directly binds integrins and, in fact, integrins that bind laminin, a uterine abundant protein. R28 deletion leads to a decrease in GAS adhesion to primary decidual stromal cells. R28 also promotes GAS adhesion to epithelial cells, endometrial, lung and keratinocytes. This may account for the prevalence of the emm28 genotype.
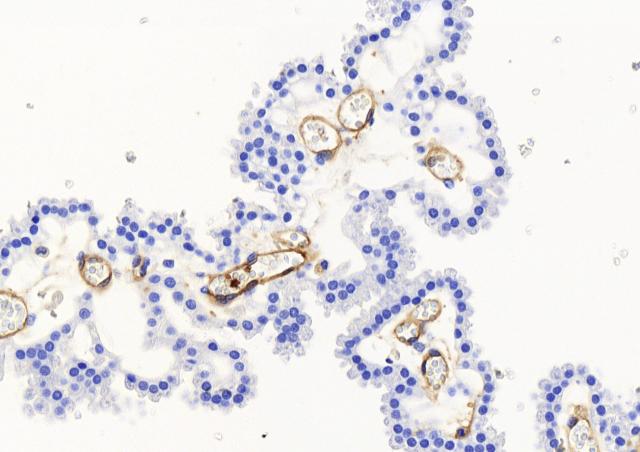
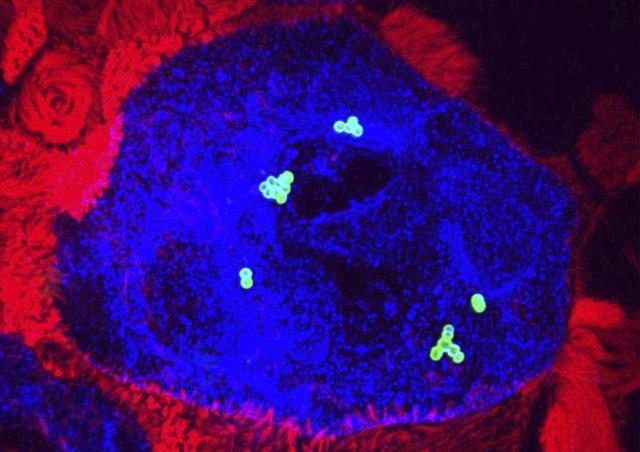
A holistic analysis of the ex vivo infection of human decidual tissue by GAS was conducted. Decidua is prepared from fetal-maternal membranes obtained after maternal consent. The study, which involved fixed and real-time imaging, image analysis tools, RNA and protein quantifications, allowed to characterize for the first time the initial steps of GAS invasive infections. GAS adheres to the tissue and multiplies on its surface in three dimensions, approximately 107-fold in 24 hours, by means of tissue secreted products. GAS invades the tissue and induces the death of stromal and immune decidual cells. GAS alters the immune response, limiting it to the immediate innate immune response. Two major GAS virulence factors, the cysteine protease SpeB and the hemolysin Streptolysin O are involved in these various processes. This accounts for the efficiency of GAS in inducing puerperal fevers.

Fatty acid metabolism and GAS virulence
In order to decipher the mechanisms used by GAS to invade tissues, we rely on a result published by other teams. Strains deficient in the transcriptional repressor of the fatty acid biosynthesis pathway (FASII), FabT, display an attenuated virulence. In addition, these strains disseminate much less, about 200-fold, than the wild-type strain. Our hypothesis is that deregulation of the FASII genes leads to defects in the fatty acid composition of the membrane. These defects would alter the positioning of surface, membrane or parietal, proteins. All these modifications would interfere with the capacity of GAS to adapt to its environment.
By analyzing the transcriptomic profiles of strains grown under various conditions, we defined the FabT regulon. A single gene, other than the FASII genes, is co-regulated with these. We established the changes in fatty acid composition of wild type and mutant strains. A lipidomic analysis shows that the mutant has a defect in lipids that are involved in the establishment of bacterial surface elements. By proteomic approach, we confirmed that surface proteins are in different quantities in the wild type and mutant strains. By approaches such as those carried out during the study of the GAS - decidua interaction, we will characterize the physiological consequences of the FabT mutation. Thus, we will determine the causes of the virulence defect in strains that do not control the expression of genes from the fatty acid biosynthesis pathway. In the long term, understanding the role of FabT in virulence will allow the use of FabT or proteins identified in the mutant as targets of specific antimicrobial compounds.