Unlike adults, children exhibit many cells that proliferate during their growth. As a result, chemotherapy and radiotherapy treatments induce significant lesions that can cause serious, sometimes fatal, late side effects. There is therefore a crucial need for new, less toxic therapies.
Previous work had shown that the DiPRO1 protein played an important role in controlling proliferation, cell differentiation and programmed cell death (apoptosis). DiPRO1 shares targets with another protein, SIX1, which is an important regulator of muscle development. It is also overexpressed in pediatric rhabdomyosarcoma and Ewing sarcoma, two mesenchymal cancers. These high levels of DiPRO1 are linked to recurrent tumors, suggesting that it is a potential prognostic marker
DiPRO1, a protein overexpressed in certain childhood mesenchymal cancers
In the present study published in the journal EMBO Molecular Medicine, the scientists compared in vitro the effects of overexpression or loss of expression of the DiPRO1 protein in healthy myoblasts and cancerous mesenchymal cells. In both cell types, they found that DiPRO1 plays a dual role in gene regulation and DNA modification. It can act as a repressor or activator of the expression of many genes, thus playing a key role in reprogramming cell fate.
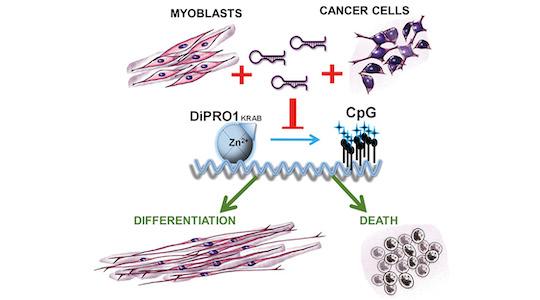
Figure legend: DiPRO1 inhibition induces epigenetic activation of CpG islands, promoting myogenesis in myoblasts while leading to apoptosis in rhabdomyosarcoma and Ewing sarcoma cells. Targeting DiPRO1 with si/shDiPRO1 nanomedicines represents a potential treatment avenue for mesenchymal cancers
DiPRO1, a potential target for future treatments
The results show that the DiPRO1 protein plays distinct roles in healthy cells and in cancer cells. In healthy muscle cells, DiPRO1 helps control genes that are important for muscle growth, preventing them from transforming into fully developed muscle cells.
The role of DiPRO1 in mesenchymal cancers is particularly interesting. Inhibiting its expression is toxic to the cancer cell and leads to cell death.
Cancers of mesenchymal origin share common mechanisms involving DiPRO1. Consequently, targeting DiPRO1 could constitute a new strategy for the treatment of these cancers. A first study, using a mouse model in which it is possible to graft tumors of human origin, showed that the use of nanomedicines to target DiPRO1 caused a significant inhibition of tumor cell growth.
Since depletion of DiPRO1 in normal cells does not induce cell death, this provides an opportunity to specifically treat cancer cells. This selective action represents a potentially less toxic approach to treating patients who suffer from significant late effects of their current cancer treatments.
Research on DiPRO1 inhibitors could lead to less harmful and possibly more effective treatments.
Reference
Rich J, Bennaroch M, Notel L, Patalakh P Alberola J, Issa F, Opolon P, Bawa O, Rondof W, Marchais A, Dessen Ph, Meurice G, Le-Gall M, Porlot M, Ser-Le Roux K, Droin N, Raslova H, Maire P, Geoerger B, and Pirozhkova I. DiPRO1 distinctly reprograms muscle and mesenchymal cancer cells. EMBO Molecular Medicine (EMM-2023-174203) Jul 15. doi: 10.1038/s44321-024-00097-z






